Manufacturing Gauze Ribbon, Disposable Combine Dressing Sterile, Foley Balloon Catheter and many more health industry products.
A.P.S. SURGICALS PRIVATE LIMITED
GST : 07AAPCA4250Q1Z4
Manufacturing Gauze Ribbon, Disposable Combine Dressing Sterile, Foley Balloon Catheter and many more health industry products.
Manufacturing Gauze Ribbon, Disposable Combine Dressing Sterile, Foley Balloon Catheter and many more health industry products.
About Us
We, A.P.S. Surgicals Private Limited, are serving renowned names like Fortis Group of Hospitals, Care Hospitals Group, Ministry of Defense (AFMSDs, Command Hospitals, Base Hospitals), AMRI Group of Hospitals, BLK Super Speciality Hospital, etc., with our quality products. Our offered items include Anti Bacterial Bed Bath Towel, Urine Collecting Bags, Standar Regular Absorbable Haemostatic Gelatin Sponge USP, etc., that are demanded in the market because of their quality. Today, we have attained the position of reputed manufacturer and exporter and are serving clients from 60 different countries like Uzbekistan, UAE, Philippines, Qatar, Egypt, Tajikistan, Indonesia and many more. We have opened offices in Tajikistan and Uzbekistan and will be inaugurating more at different locations throughout the globe.
The business operations of our company are handled by the best team of professionals having rich experience and knowledge. With their support, we are running our venture successfully and manufacturing more than 3000 products. We also have a record of attaining 100% client satisfaction in all our deals and this is enabling us to attract new clientele as well.
Before initiating supply, the products are tested on various parameters to ensure they are worthy. Throughout our production process, we maintain the complete traceability of all the materials used. This effectiveness of quality management systems enables us to bring the best for clients and deliver the best to them.
Why Choose Us?
- Manufacturing and Experience: We have good experience in this industry and have the best production facilities installed at our unit. We adhere to Good Manufacturing practices as well as WHO Standards
- Quality Policy: We maintain high standards of quality in our products and make sure to check the products on multiple parameters before dispatch.
- Serving Globally: With the help of our global partners, we deliver our products in different countries within the promised time frame.
Certifications
We have received certificates like ISO 9001:2015, ISO 13485:2016, ISO 14001:2015, CE, WHO-GMP and many more certifications on the basis of quality of Urine Collecting Bags, Anti Bacterial Bed Bath Towel, Standar Regular Absorbable Haemostatic Gelatin Sponge USP, etc.
Infrastructure: Manufacturing Unit
To attain world-class standards in our offerings, we take assistance from the best production machines and tools. We take help from laser cutting machines for gauze dressings into various shapes and sizes. Further, the products produced are being packed in DuPont Tyvek paper with the help of a custom-built automated packaging machine. All the operations at our unit are handled by well-trained, skilled and dedicated professionals. Our taskforce has been imparted training by experts from Korea, Germany, and USA.
Sterilization
As a manufacturer, we understand the importance of using sterilized products and thus, offer the same to our customers. The process of sterilization cannot be skipped as the infected product range can trigger the infection and lead to serious results. To carry out the process of sterilization, we use Ethylene Oxide (EO or ETO), Electron beam (E-Beam), Gamma, X-ray and Nitrogen Dioxide (NO2) technologies.
How Can Our Expertise Help You?
- Customers will get customized solutions that are safe and effective
- We have a global network of facilities located at evenly distributed locations
- High-quality end processes that meet all the safety standards
- Advisors will assist in solution development, validation as well as in testing
- Prompt product processing services
- One-stop partner for all the sterilization modality requirements and provides access to the best-in-class products
Modalities Used To Sterilize Medical Devices
- EO offers material compatibility except for moisture and temperature-sensitive material. This is an effective sterilization modality for manufacturers who produce radiation-sensitive products.
- Gamma offers polymer compatibility with some limitations because of oxidation effects. The product can be dispatched for delivery as soon as it passes the Gamma irradiation facility.
- E-beam offers polymer compatibility but with limitations due to oxidation. It is ideal for manufacturers that produce sterile products with low density or product packaged in small and uniform packages
- X-ray offers a wide range of polymer compatibility with some limitations due to oxidation effects. It is ideal for manufacturers producing products of high density, in large volumes.
- NO2 based sterilization offers ultra-low temperature, no cytotoxic residuals, minimal pressure and fast cycle times.
Clean Room
We own the facility of a clean room, where the environment is controlled as per the requirement. We have listed below some remarkable features of our advanced cleanroom. Some of the remarkable operational and constructional features of our clean room are:
- It is designed to meet the class 10,000 cleanliness standard of US FED 209E.
- The air handling systems are intended to ISO requirements (ISO 14644), thus supplying the air of high quality. The processing areas receive filtered air with a minimum of 60 air changes/hour.
- The process area has terminal High-Efficiency Particulate (HEPA) filters with an efficiency of 99.97% down to 0.3 microns.
- The airflow is unidirectional and laminar fashion across all workstations, thereby, reducing the risk of contamination.
- Between adjacent rooms, a differential pressure of 12 to 15 Pascals is preserved to avoid ingress of particulates.
- Temperature, as well as relative humidity, is controlled via air conditioning systems and dehumidifiers systems.
- The clean rooms are assembled using non-particle shedding modular panels made from powder coated GI sheets and polyurethane foam at its center, ensuring airtight clean rooms. Also, epoxy flooring and coverings give smooth surfaces plus protect against the accumulation of dust.
- Raw material movement in this room is carried out through well-designed airlocks and dynamic pass boxes.
- All doors and windows are flushed with walls that are unified with non-particle shedding gaskets to make the clean room airtight.
- The door Interlocking System is there to avoid cross-contamination and to prevent the unauthorized man-material entrance.
-

Sterile Dressing Kit -

Standar Regular Absorbable Haemostatic Gelatin Sponge USP -

Chlorhexidine Gluconate Antibacterial Gauze Dressing -
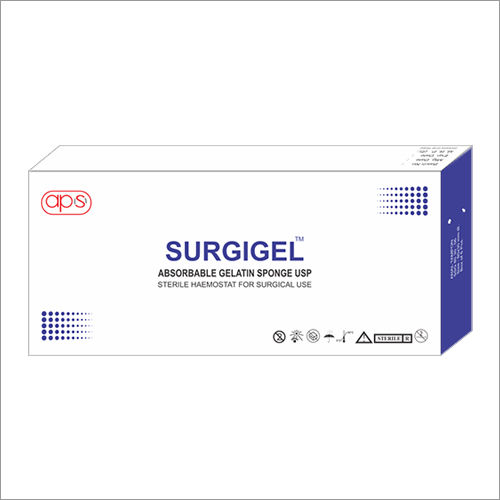
Absorbable Gelatin Sponge USP -
Dental Cubes Absorbable Gelatin Sponge USP -

Disposable Combine Dressing Pad -

Sterile Gauze Swab -

Absorbable Haemostat Oxidised Regenerated Cellulose Sponge -
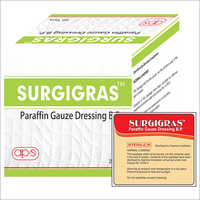
Paraffin Gauze Dressing BP -
Disposable Sterile Abdominal Sponges -

Gauze Ribbon -

Gamjee Dressing Pad -

Gamjee Roll -

Elastic Adhesive Bandage Cannula Fixator B.P -

Non Sterile Gauze Swab -

Plaster Of Paris Bandage B.P -

Non Sterile Abdominal Sponges -
Transsurg - Blood Administration Set -
Infusion Set -

Cotton Crepe Bandage -

Sterile Surgical Gloves -

Latex Examination Gloves -

Urine Collecting Bags -
Urine Collection Bag With Measured Volume Meter -

Microporous Hypoallergenic Paper Tape -

Elastic Adhesive Bandage -

Foley Balloon Catheter -
Umbilical Cord Clamp -

Anti Bacterial Bed Bath Wipes -

Anti Bacterial Shampoo Wipes

 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese